"ATP"
|
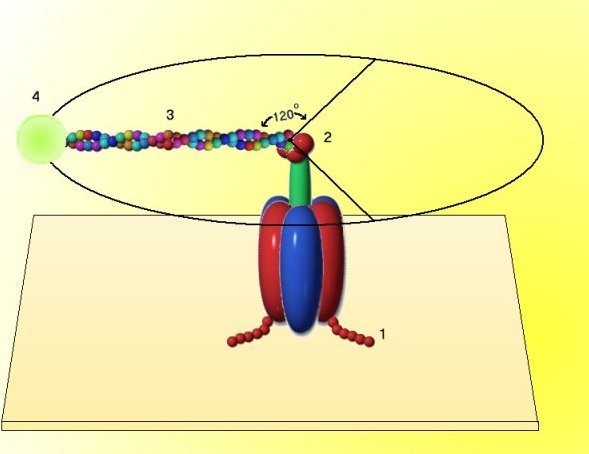
(1) The F1
hexamer was anchored to a glass slide by attaching 6 histadine residues (# 1) to each b subunit.
(2) The glass slide was coated with a metal reagent that binds histadine, and the b subunit was attached to the slide.
(3) The g unit (# 2) was modified so that it would bind a molecule of actin (# 3), a long, linear protein of muscle.
(4) The actin molecule in turn, was modified by attachment of a molecule that fluoresces in ultraviolet light (# 4). Thus the movement of the g unit could be monitored directly in the light microscope with a uv light source, even though the whole assembly is exceedingly small.
(5) When ATP was added to the anchored F1 subunit, the fluorescent marker was seen to rotate in discrete 120 degree increments. During the synthesis of ATP, rotation of the g unit by the proton motive force acting on the F0 complex would therefore generate ATP. [after H. Noji et al., 1997, Nature 386:299; and R. Yasuda et al., 1998, Cell 93:1117]