 |
|
||
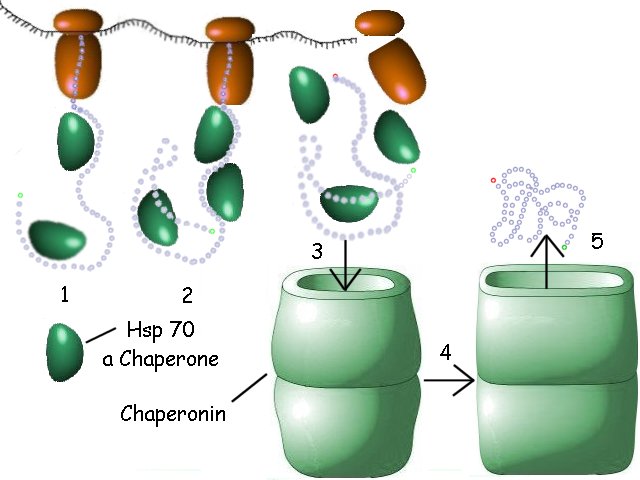
Many cellular proteins require help in achieving their active conformation or shape. This help is supplied by a class of cytoplasmic proteins called chaperones and another group of protein aggregates (16 molecules in the bacterial form) called chaperonins. They function as follows:
1. The growing peptide is coated with a chaperone protein such as Hsp70 (dark green) that prevent the premature formation of incorrect associations within the molecule by blocking interactive groups.
2. As the protein continues to elongate, more of the Hsp70 binds.
3. When the protein is complete, it is released from the ribosome. It may then interact with itself over a period of time as the chaperones gradually disperse. If a chaperonin is required for its proper folding, the protein enters the chaperonin, losing its coating of Hsp70 in the process.
4. Within the chaperonin, the protein is sequestered in a controlled environment and is also protected from interacting with other proteins in the cytoplasm while it assumes its correct final conformation.
5. When the protein has achieved its correct folded state, the chaperonin hydrolyzes ATP, changes its shape into a more relaxed conformation, and the correctly folded protein leaves the chaperonin.
 Return
Return