"Prebiotic Synthesis of Adenine and Adenosine triphosphate (ATP)"
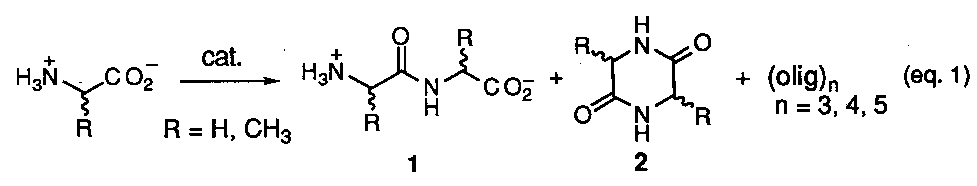
The prebiotic synthesis of adenine, a key structural component in ATP and one of the cornerstone bases in DNA and RNA, might arguably be considered one of the most crucial in any chemical evolutionary proposal due to the importance of these molecular assemblies to all living systems as we know them today. The most cited references for the likely prebiotic synthesis of this molecule is the work of Oro and coworkers1 which involves heating concentrated solution of ammonium cyanide in water (eq 1).

Reported yields of adenine were less that 1% with the remainder of material being composed of a dark tarry residue resulting from the polymerization of hydrogen cyanide (HCN). Although a mechanistic scheme was originally proposed to explain the union of five molecules of HCN into the purine structure via a trimeric intermediates (e. g. aminomalononitrile (2), subsequent studies2, have shown that the tetramer, cis-diaminomaleonitrile (3) is the first stable isolatable intermediate formed (eq 2).
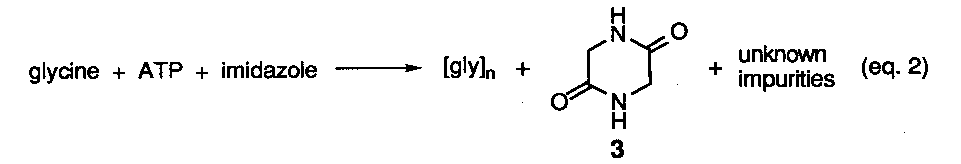
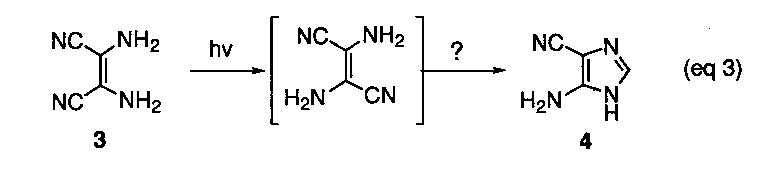
The dimer, iminoacetonitrile (1), and trimer 2 have been shown to be the precursor to the tetramer and are not stable under the reaction conditions. Further conversion of the tetramer into 4-aminoimidazole-5-carbonitrile (4) has been demonstrated in separate photochemical experiments, although the mechanism for this unusual transformation has never been specified (eq 3).
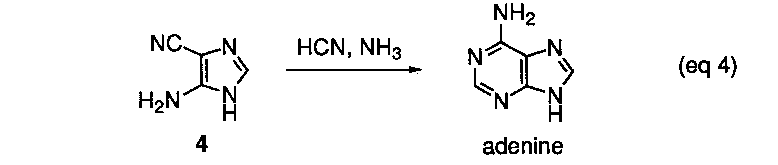
The conversion of 4 into adenine by reaction with formamidine has been demonstrated in isolated experiments (eq 4).
However, the thermal conversion of the cis-tetramer into the requisite imidazole 4 has. not been experimentally demonstrated. The mechanistic details of such a process is of major importance to the proposed overall prebiotic scheme. It brings into question whether the one-pot conversion of HCN into adenine has been adequately demonstrated as a continuous linear sequence.3 It is our objective to examine alternative mechanistic proposals that can be designed to test the viability of the conversion of 3 into 4 under thermal and/or photochemical reaction conditions. Furthermore, a reexamination on the exact structure of the proposed compound isolated from the original studies is warranted.
1. Oro, J. "Synthesis of Adenine from Ammonium Cyanide" Biochem. And Biophy. Res. Comm. 1960, 2, 407. Oro. J.; Kimball, A. P. "Synthesis of Purines Under Possible Primitive Earth Conditions" Arch. Biochem. Biophys. 1962 96, 293.
2. Sanchez, R. A.; Ferris, J. P.; Orgel, L. E. "Studies in Prebiotic Synthes~s. II. Synthesis of Purine Precursors and Amino Acids for Aqueous Hydrogen Cyanide." J. Mol. Biol. 1967, 30, 223. Sanchez, R. A.; Ferris, J. P.; Orgel, L. E. "Studies in Prebiotic Synthesis. IV. Conversion of 4-Aminoimidazole-5-carbonitdle Derivatives to Purines."J. Mol. Biol. 1968, 38, 223.
3.For a critical analysis concerning the prebiotic origin of adenine, see: Shapiro, R. Origins of Life and Evol. Of the Biosphere 1995, 25, 83.