 |
|
||
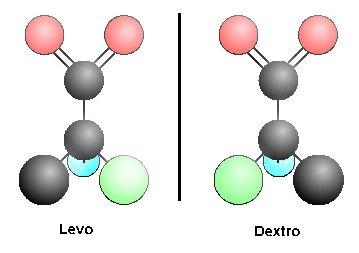
Figure 1. Two mirror image (enantiomeric or stereoisomeric) forms of the same molecule cannot be superimposed without breaking a covalent bond. The molecules are designated Levo (left or l-) or Dextro (right or d-) for the direction that each rotates polarized light passed through a solution of the compound. The two forms are identical in chemical properties, but different in physical properties, as a result of the positioning of atoms about the optically active center, in this case, an asymmetric carbon atom. It is asymmetric because all four bonds connect to different chemical groups or atoms, and as a consequence of the tetrahedral structure of the carbon atom, mirror image molecules are possible. When this compound is synthesized in the laboratory, the synthesis results in equal quantities of right-handed and left-handed forms (a racemic mixture). In living systems, only the l-forms of amino acids and the d- forms of sugars are used. Biological systems and biologically-derived systems (enzymes) can distinguish enantiomers, whereas chemical and many physical processes cannot distinguish them. With the passage of time, thermal activity can cause bonds to be broken such that the single form (in biological systems, l-amino acids) is gradually converted to a racemic (equal d- and l- forms) mixture. Ó 2010 Arthur V. Chadwick, Ph.D.