 |
|
||
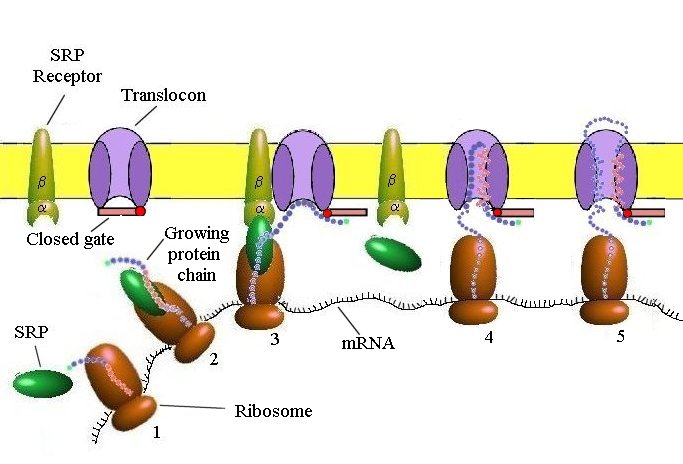
1. The Signal Receptor Particle (SRP)recognizes and binds to the emerging signal anchor sequence (red) of the nascent (growing) protein and integrates into the ribosome.
2. The integrated SRP engages a complex SRP receptor (alpha and beta proteins) in the membrane of the endoplasmic reticulum.
3. When thus anchored, the hydrolysis of GTP (Guanosine triphosphate) triggers the release of the SRP and the SRP receptor from the ribosome, opens the gate blocking the translocon, and inserts the nascent protein chain into the translocon. The translocon is a membrane complex made up of three or four sec61 complexes, each composed of three proteins (sec61 alpha, beta and gamma) The alpha protein is a membrane-spanning protein, making ten membrane crossings. Another major component of the translocon is the TRAM protein, which crosses the membrane at least eight times.
4. The ribosome is now anchored to the translocon by the growing peptide. As the peptide elongates, the helical signal-anchor sequence (red) extends through the membrane to the inner lumen.
5. When a second helical series of amino acids, the Stop-Transfer Membrane-Anchor Sequence (blue) is encountered, the movement of the growing peptide chain into the translocon ceases.
 Next
Next