Abiogenic
Origin of Life: A Theory in Crisis
” 2005 Arthur V. Chadwick, Ph.D.
Professor of Geology and Biology
Southwestern
Origin of Life Research as Science:
††††††††††† All phenomena are essentially unique and irreproducible. It is the aim of the scientific method to seek to relate effect (observation) to cause through attempting to reproduce the effect by recreating the conditions under which it previously occurred. The more complex the phenomenon, the greater the difficulty encountered by scientists in their investigation of it. In the case of the scientific investigation of the cause of the origin of life, we have two difficulties: the conditions under which it occurred are unknown, and presumably unknowable with certainty, and the phenomenon (life) is so complex we do not even understand its essential properties. Thus, at the outset, there is a condition of "unknowableness" about the origin of life quite different from that associated with most scientific investigations. Since the methods of science and the normal rigors of scientific investigation are handicapped, one should be more willing to give serious consideration to information from any source that might contribute to our understanding of origins, in particular to the hypothesis that life was created by an infinitely superior Being. If a careful scientific analysis leads us to conclude that the proposed mechanisms of spontaneous origin could not have produced a living cell, and in fact that no conceivable natural process could have resulted in the spontaneous origin of life, the alternative hypothesis of Creation becomes the more attractive. If, on the other hand, we find the proposed mechanisms to be plausible, we must be aware that the methods of science can never answer with certainty the question of origin. We will begin with a consideration of the nature of life. Then we will attempt to evaluate the current state of affairs in origin of life research, sometimes referred to as "paleobiogeochemistry."
What is Life?
Cells are the simplest element of living things. All complex organisms are composed of cells. Yet cells as we know them today are exceedingly complex. Within each single cell resides a microcosm of the entire organism of which it is a functional part. Single cells that are free-living such as amoebae or protozoa must carry on within that single cell all of the functions carried on by entire organisms, and individual cells have digestive, reproductive, respiratory, nervous, skeletal, excretory, muscular, etc. systems on a minute scale that are exceedingly complex. In addition, all living cells share a suite of common features that can be assumed to be fundamental to life. These include, the genetic code, the information-rich primary DNA code, the DNA polymerase required for replicating that code, RNA intermediates and the RNA polymerases required for transcribing the DNA, the mechanism of protein synthesis involving the ribosome, the transfer RNAís and the enzymes required to attach the correct amino acids to the correct transfer RNA, the cell membrane, and metabolic pathways required to generate the materials necessary for the above reactions involving hundreds of enzymes. Perhaps the first living cell did not have the capacity to carry out the activities of even the simplest modern living cell. In that case we must decide what features can be eliminated, and having eliminated these, how the cell could survive. We must then explain how these features arose later in the process of evolution and why they are common to all cells. We will say more about these features later. For now, let us take a look at the system proposed by naturalists to have given rise to this cell.
How do Abiogenic
Origin of Life Proponents Think it Happened?
Four components are essential to the story of the origin of life put forward by proponents of naturalism.
1. An atmosphere full of reduced gas molecules and an energy source to convert these molecules into the biological precursors required for life.
2. An ocean full of the small biological molecules that result.
3. A mechanism to generate from this ocean of molecules the kinds of information-rich polymers necessary for a living cell.
4. A belief that if step 3 can be implemented, it will result almost inevitably in the formation of a living cell.
We will examine each of these in turn.
Chemical Evolution:
††††††††††† The earliest serious studies on origin of life date to the 1920's when J.B.S. Haldane and A. I. Oparin independently suggested that life had originated spontaneously from non-living matter on the earthís surface at some time in the distant past, and provided a scenario for its occurrence. Since, at that time, the view prevailed that life was nothing more than complicated chemistry, their ideas became widely accepted among those seeking to establish a naturalistic origin for life on the earth. It was not until 1953, when Stanley Miller did his now-famous experiments using the reducing environment proposed by Oparin (CH3, NH4, H2O and H2) in a glass apparatus energized by a Tesla coil, that the spontaneous origin idea gained scientific acceptability. In this apparatus Miller generated a variety of simple compounds including a few amino acids, as well as a quantity of "tar" (polymerized organic sludge of no interest to paleobiogeochemists). Miller and Urey went on to propose that ultraviolet (UV) light, corona discharge and lightning produced small biological precursor molecules on the "primitive earth," which subsequently were deposited in the oceans by the hydrologic cycle. Carl Sagan proposed the "primitive earth" was subjected to UV flux 100 times the present level, and that H2S from volcanism was the agent catalyzing the transfer of energy from the UV light to the UV transparent elements in the atmosphere. In the early 1970's Bar-Nun demonstrated high velocity shock waves to be 10,000 times as efficient as other methods at converting the gaseous reducing atmosphere of Oparin to small molecules, forming four amino acids. More recently, under somewhat suspect conditions, purines and pyrimidines, the kinds of bases contained in DNA and RNA, were reported to have been made by Yuasa et al. (84). At present 14 of the 20 amino acids can be made under the reducing conditions proposed to exist on the "primitive earth". Unfortunately for spontaneous origin of life enthusiasts, the preponderance of the amino acids produced by these experiments are either glycine or alanine, the two simplest amino acids, and many non-proteinous amino acids are produced that will compete with the 20 proteinous amino acids in any abiological reactions.
††††††††††† Many other problems exist, but for those people who want to believe in the spontaneous origin of life, the mere articulation of a model to produce the "hot dilute soup" of Haldane and Oparin or the "dilute chicken soup" of Sagan, no matter how unsatisfactory it may be, has encouraged them to believe it can explain the origin of life. We will walk through the modern synthesis of origin of life speculations, then will attempt to evaluate these speculations within the parameters the investigators have set for themselves, to see what hope they offer of achieving the end intended, ie. the spontaneous origin of a living cell. We will begin by considering the earthís early atmosphere and the likelihood that it could generate an ocean full of biological useful molecules. Then we will go on to consider whether we could produce the kinds of biologically important polymers needed for life, given an ocean full of small molecules. We will then ask whether it is even possible to make a living cell and investigate some significant areas of molecular biology to identify the complexity a living system entails.
Evidence for a Reducing Atmosphere:
††††††††††† Oparin
first suggested an atmosphere for the "primitive earth" of H2,
H2O, NH3, CH4 largely
because he, being a chemist, recognized that such a "reducing"
atmosphere would be required chemically to produce the "hot dilute
soup" from which he believed life originated. Being a chemist, he also
recognized the necessity to exclude oxygen or oxidizing compounds from the
mixture. It was convenient, then, that such a mixture proved capable of
generating a variety of small molecules of biological interest. The real
question is "did such an atmosphere ever exist on the earth?" A
careful analysis from geological, cosmological, and chemical viewpoints reveals
that such a reducing atmosphere, if it ever existed, would have been short
lived. J. C. G. Walker states "The strongest evidence [for a reducing
atmosphere] is provided by conditions [required] for the origin of life. A
reducing atmosphere is required" (
††††††††††† Data against a reducing atmosphere have been accumulating over the past twenty years. Many who in the past considered a reducing atmosphere an absolute requirement are taking a second look. Many theoretical considerations require that the atmosphere have come from outgassing of the mantle, and such gasses today are uniformly oxidized. The likelihood of a neutral atmosphere (CO2, H2O, N2, and possibly a trace of H2) has now been conceded by most workers in this area. Such a prospect does not appear to have dimmed the enthusiasm of most workers appreciably. However the presence of free oxygen precludes virtually all scenarios thus far proposed for abiogenesis of living forms, and such an atmosphere appears at present a virtual certainty.
Evidence for the "Chicken Soup":
††††††††††† A number of careful analyses of the Oparin-Haldane scenario to generate an ocean full of small biological precursor molecules, have left gaping holes in the concept of a "dilute soup" ocean on the primeval earth. When pen is put to paper in calculating just how many molecules could result under ideal conditions, the likelihood of such an ocean vanishes. H. E. Hull (1960), L. G. Sillen (1965) and R. Shapiro (1986) have all concluded that the term "dilute" is a gross exaggeration and that the presence of even the most abundant amino acids would not have exceeded .0001 gram per liter, much too dilute to be involved in polymeric reactions required to make proteins. H. R. Hulett (1969) saw .000001 g/l as more realistic for glycine, the most abundant amino acid. K. Dose suggested .00001 g/l. Present concentrations in the mid-Atlantic range between .00001 and .0001 g/l! If the synthesis of small molecules from a gaseous primitive earth atmosphere did take place, then just as in the experimental vessels, large amounts of tarry residue inevitably resulted, so on the "primitive earth" there should have been large amounts of non-biologically produced nitrogenous tarry material that would have been incorporated into the early Precambrian sediments. No such non-biological tarry material is known in the geologic record. Thus again, we must conclude that we have no evidence that the "dilute chicken soup" ocean ever existed. It is wishful thinking that allows it to survive. As one proponent testifies, "the record of biological evolution manifest in the chemistry of living organisms....probably provides the most compelling evidence for a period of chemical evolution early in Earth history." This clearly is a tautology.
††††††††††† Numerous authors support the absence of the "hot dilute soup." A. G. Cairns-Smith (1982), W. Day (1984), H. D. Pflug (1984), C. R. Woese (1979), Hulett (1969), Shapiro (1986), M. Delbruck (1986), most of whom probably subscribe to some sort of prebiological origin of life, all conclude there is no evidence of the process having occurred. In spite of this, an equal number of authors regard the origin of life scenario as so well established that it needs no justification! Sagan and M. J. Newman have even gone so far as to declare, "The absence of evidence is not evidence of absence." For those of us who believe that life could not have originated from a non-existent "hot dilute chicken soup," such religious statements of irrationality by the likes of Sagan added strength to our conviction that he is just wrong!
††††††††††† Thus far, we have only dealt with the matter of small molecules. We have concluded that the earth did not have a reducing atmosphere, and that even if it did, there is no chance that it gave rise to the ocean full of small molecules that proponents of prebiological evolution require to make the first cell. But we have considerable ground still to cover. So let us grant the existence of an ocean full of small molecules and see what can be done with it.
The Emergence of Information-Rich Biopolymers:
††††††††††† Given an ocean full of small molecules of the types likely to be produced on a prebiological earth with the types of processes postulated by origin of life enthusiasts, we must next approach the question of polymerization. This question poses a two edged sword: we must first demonstrate that macromolecule synthesis is possible under prebiological conditions, then we must construct a rationale for generating macromolecules rich in the information necessary for usefulness in a developing precell. We shall deal with these separately.
††††††††††† The synthesis of proteins and nucleic acids from small molecule precursors represents one of the most difficult challenges to the model of prebiological evolution. There are many different problems confronted by any proposal. Polymerization is a reaction in which water is a product. Thus it will only be favored in the absence of water. The presence of precursors in an ocean of water favors depolymerization of any molecules that might be formed. Careful experiments done in an aqueous solution with very high concentrations of amino acids demonstrate the impossibility of significant polymerization in this environment. A thermodynamic analysis of a mixture of protein and amino acids in an ocean containing a 1 molar solution of each amino acid (100,000,000 times higher concentration than we inferred to be present in the prebiological ocean) indicates the concentration of a protein containing just 100 peptide bonds (101 amino acids) at equilibrium would be 10-338 molar. Just to make this number meaningful, our universe may have a volume somewhere in the neighborhood of 1085 liters. At 10-338 molar, we would need an ocean with a volume equal to 10229 universes (100, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000, 000) just to find a single molecule of any protein with 100 peptide bonds. So we must look elsewhere for a mechanism to produce polymers. It will not happen in the ocean.
††††††††††† Sidney Fox, an amino acid chemist, and one of my professors in graduate school, recognized the problem and set about constructing an alternative. Since water is unfavorable to peptide bond formation, the absence of water must favor the reaction. Fox attempted to melt pure crystalline amino acids in order to promote peptide bond formation by driving off water from the mix. He discovered to his dismay that most amino acids broke down to a tarry degradation product long before they melted. After many tries he discovered two of the 20 amino acids, aspartic and glutamic acid, would melt to a liquid at about 200oC. He further discovered that if he were to dissolve the other amino acids in the molten aspartic and glutamic acids, he could produce a melt containing up to 50% of the remaining 18 amino acids. It was no surprise then that the amber liquid, after cooking for a few hours , contained polymers of amino acids with some of the properties of proteins. He subsequently named the product proteinoids. The polymerized material can be poured into an aqueous solution, resulting in the formation of spherules of protein-like material which Fox has likened to cells. Fox has claimed nearly every conceivable property for his product, including that he had bridged the macromolecule to cell transition. He even went so far as to demonstrate a piece of lava rock could substitute for the test tube in proteinoid synthesis and claimed the process took place on the primitive earth on the flanks of volcanoes. However, his critics as well as his own students have stripped his credibility. Note the following problems:
1) Proteinoids are not proteins; they contain many non-peptide bonds and unnatural cross-linkages.
2) The peptide bonds they do contain are beta bonds, whereas all biological peptide bonds are alpha.
3) His starting materials are purified amino acids bearing no resemblance to the materials available in the "dilute soup." If one were to try the experiment with condensed "prebiological soup," tar would be the only product.
4) The ratio of 50% Glu
and Asp necessary for success in these experiments bears no resemblance to the
vastly higher ratio of Gly and
5) There is no evidence of information content in the molecules.
All of his claims have failed the tests of rationality when examined carefully. As promising as his approach seemed in theory, the reality is catastrophic to the hopes of paleobiogeochemists.
††††††††††† A number of other approaches have been tried. The most optimistic of these is the use of clays. Clays are very thin, very highly ordered arrays of complex aluminum silicates with numerous other cations. In this environment, the basic amino groups tend to order and polymers of several dozen amino acids have been produced. While these studies have generated enthusiastic interest on the part of prebiological evolutionists, their relevance is quickly dampened by several factors.
1) While ordered amino acids joined by peptide bonds result, the product contains no meaningful information.
2) The clays exhibit a preference for basic amino acids.
3) No polymerization of amino acids results if free amino acids are used.
4) Pure activated amino acids attached to adenine must be used in order to drive the reaction toward polymerization. Adenylated amino acids are not exactly the most likely substrate to be floating about the prebiological ocean.
5) The resultant polymers are three dimensional rather than linear, as is required for biopolymers.
At least one optimistic scientist (Cairns-Smith, 1982) believes that the clay particles themselves formed the substance of the first organisms! In reality, the best one can hope for from such a scenario is a racemic polymer of proteinous and non proteinous amino acids with no relevance to living systems.
††††††††††† A final chapter has recently been opened with the discovery of autocatalytic RNA molecules. These were originally received with great excitement by the prebiological evolutionists because they gave hope of alleviating the need to make proteins in the first cell. These so-called "ribozymes" proved incapable of rising to the occasion, however, for not only are the molecules themselves very limited in what they have been shown capable of doing, but the production of the precursors of RNA by any prebiological mechanism considered thus far is a problem at least as difficult as the one ribozymes purport to solve:
1) While ribose can be produced under simulated prebiological conditions via the formose reaction, it is a rare sugar in formaldehyde polymers (the prebiological mechanism believed to have given rise to sugars). In addition the presence of nitrogenous substances such as amino acids in the reaction mixture would prevent sugar synthesis (Shapiro, 1988). Cairns-Smith (1993) has summarized the situation as follows:"Sugars are particularly trying. While it is true that they form from formaldehyde solutions, these solutions have to be far more concentrated than would have been likely in primordial oceans. And the reaction is quite spoilt in practice by just about every possible sugar being made at the same time - and much else besides. Furthermore the conditions that form sugars also go on to destroy them. Sugars quickly make their own special kind of tar - caramel - and they make still more complicated mixtures if amino acids are around."
2) When produced and condensed with a nucleotide base, a mixture of optical isomers results, only one of which is relevant to prebiological studies.
3) Polymerization of nucleotides is inhibited by the incorporation of such an enantiomorph.
4) While only 3'-5' polymers occur in biological systems, 5'-5' and 2'-5' polymers are favored in prebiological type synthetic reactions (Joyce and Orgel, 1993, but see Usher,et. al. for an interesting sidelight).
5) None of the 5 bases present in DNA/RNA are produced during HCN oligomerization in dilute solutions (the prebiological mechanism believed to give rise to nucleotide bases). And many other non-coding bases would compete during polymerization at higher concentrations of HCN.
In addition to the problems of synthesis of the precursors and the polymerization reactions, the whole scheme is dependent on the ability to synthesize an RNA molecule which is capable of making a copy of itself, a feat that so far has eluded strenuous efforts. The molecule must also perform some function vital to initiating life force. So far all of this talk of an "RNA World" remains wishful thinking best categorized as fiction. The most devastating indictment of the scheme however, is that it offers no clue as to how one gets from such a scheme to the DNA-RNA-Protein mechanism of all living cells. The fact that otherwise rational scientists would exhibit such rampant enthusiasm for this scheme so quickly reveals how little faith they have in all other scenarios for the origin of life, including the ones discussed above.
Investigator Interference, Proximity and Stereoisomers:
††††††††††† In attempting to establish the credibility of various models for the origin of life on earth, I have neglected certain considerations of overriding importance in order to enable experiments to be analyzed on their own merits. But now we can no longer ignore these considerations.
††††††††††† In all experimental studies on the origin of life, the presence of the investigator makes a significant contribution to the conclusions and to the conditions of the experiment itself. When the investigator sets out to achieve a certain objective (synthesis of precursors or polymerization of precursors) he or she naturally seeks to define a system with some possibility of achieving the desired end. Thus conditions are chosen in which some of the materials are appropriate for a prebiological earth, giving the studies an air of credibility. The remaining conditions are carefully crafted to achieve the desired end. Thus the reader is left with the impression that many things would have been possible on the prebiological earth that have no probability whatsoever. For example, when Fox performed his experiments to make proteinoids from amino acids using lava rock instead of a glass test tube, he gave the impression that this was a plausible model for the prebiological earth. What he was careful to avoid emphasizing was that he was carrying out the reaction on the hot lava with a mixture of purified crystalline amino acids produced by biological organisms (soy beans), and purified by other biological organisms (man). He was also himself carefully controlling the temperature and time and exposure to water. I leave it to you to determine what would be the result of such a study carried out on a hot lava rock with condensed prebiological soup.
††††††††††† The same criticism can be made of every other study mentioned to date, from Millerís original classic study using a glass enclosed mixture of purified refluxing gases, to studies on layered smectite clays using purified mixtures of adenylated amino acids. Most of these studies have been designed to obtain a desired outcome, not to test the conditions the investigators themselves believe to be present on the prebiological earth. Yet the results are used to reinforce the validity of the abiotic earth they did not test. Even those that have sought to achieve abiotic conditions cannot preclude the influence of the investigator. After a careful review of the abiogenic research scene, J. Brooks and G. Shaw (1973) concluded:
"These experiments...claim abiotic synthesis for what has in fact been produced and designed by highly intelligent and very much biotic man."
††††††††††† Such candidness is refreshing, honest and long overdue.
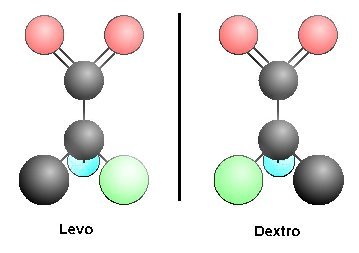
††††††††††† Another equally serious and pervasive problem is that of enantiomers or stereoisomers. This problem, perhaps more than any others, foils the efforts of all prebiologic investigators to achieve ultimately meaningful results. Any carbon-containing compound with four different groups attached creates a center of asymmetry, permitting a sister molecule to exist with the same constituent groups in a mirror image configuration. The two compounds thus formed have identical chemical properties, and can generally only be separated from their optical twin using biological systems as filters.
|
††††††††††† Such stereoisomers are a difficulty for origin of life theorists and experimentalists for the following reasons. In living systems, one and only one of the two stereoisomers is used, and for amino acids it is always the l- form, for sugars it is the d- form. But when molecules are synthesized in the laboratory or under conditions believed to exist on the prebiological earth, both isomers are formed in equal quantities. How then can we explain the predilection to choose only one isomer, and the l- isomer for all 20 amino acids when the chemical properties are identical? Great effort has been invested in trying to circumvent this problem with no success. It was hoped that possibly clays would discriminate stereoisomers, but they donít. This is a difficulty that cannot be dismissed. Unless an explanation for the choice of stereoisomers can be developed, the only solution to the problem is what I believe is the correct solution in any case: it was designed.
††††††††††† An equally difficult challenge to any of the schemes to generate informational polymers, whether protein or nucleic acid is the presence of competing reactants. Estimates vary, but Gould et al. (1981) suggests there may have been ten times as many non-proteinous amino acids as the twenty that are involved in protein (20). With little or no control over which reactions will occur, the presence of competing species, either non-proteinous amino acids or non-coding bases will wreak havoc on any systematic development of informational molecules. Furthermore, if we are successful for a time at using the correct bases or amino acids, we are only increasing the relative concentrations of the competing species, making the problem worse with time. Any calculations of probability of generating anything will be vastly underestimated because of the inability to quantify, and thus to include the contributions of these elements.
††††††††††† A third issue I call
"the proximity problem." If a certain number of molecules are
required in order to produce a living cell, producing one of these molecules in
the Indian Ocean and another in the
Additional lines of evidence indicate that life would have had to originated in an extremely short time. Seawater is thought to circulate through hydrothermal vents at such a rate that the entire ocean would pass through every 10 million years. Temperatures at hydrothermal vents can reach 350 degrees C. At this temperature, organic compounds of all sorts would be rapidly degraded. For example, the sugar ribose, so vital in some of the origin of life schemes, decomposes with a half-life of less than 50 years at 0 degrees C, and of only slightly over an hour at 100 degrees C. Adenine, the principal base in living systems, involved not only in the storage and application of information, but also in cellular energetics, has a half-life of only 204 days at 100 degrees C. The minimum cellular genome is estimated by various techniques at about 562,000 nucleotide pairs, close to the 580,000 nucleotide pairs of Mycoplasma genitlium, the simplest life form known today. The limited time available for the complex metabolic processes of life to arise before their components were degraded contrasts sharply with the complexity of the simplest living system (Lazcano and Miller 1996).
Can life originate from a "hot dilute soup"?
††††††††††† What is life? This is an important question. If there is a continuum from nonliving to living in the present world, then perhaps it would not be too difficult to visualize nonliving things slipping across the boundary. What would constitute a living cell? What criteria must be met?
††††††††††† 1) It must have integrity--it must be a self-contained entity.
††††††††††† 2) It must be bounded--it must have a container that is part of the entity.
††††††††††† 3) It must be capable of reproducing itself, contents and container.
††††††††††† 4) It must be capable of importing materials and energy.
††††††††††† 5) It must be capable of making molecules not derivable from its environment.
††††††††††† The list could be extended indefinitely, but for now we will stop with some of the more critical properties. Letís analyze the third, that of reproduction. What is the minimal level of information required to enable the reproduction of a cell? Where did that information come from? Various approaches can be taken to this question. Probably the most valid approach comes from a study of the least complex of all free-living organisms. Such studies yield estimates of from several hundred thousand to several million bits of information (i.e. 100,000-1,000,000 nucleotides). Others insist a reductionist empirical approach is more rational--what does a cell need to reproduce, as an absolute minimum. Various estimates depend largely on the state of optimism of the author involved, but except for cases of extreme naivete, the estimates focus on about 100 proteins with specific functions in either replication, transcription, or translation. Making the proteins themselves in a reproducible form requires complex information, and that information must have been available first, in the form of DNA or RNA. But since the information content of the DNA or RNA approximates that of the proteins produced from the DNA/RNA, the problems are similar in either case.
††††††††††† Because the case is clearer, we will first consider the problems associated with creating a protein, and particularly, a single protein, cytochrome c. Cytochrome c will be a useful example since it is widely distributed in nature and is the most thoroughly sequenced protein. Because it is present in virtually all organisms, it would have to have been among the first cellular proteins. Cytochrome c consists of a sequence of about 110 amino acids and cytochrome c from over 100 organisms have been sequenced . Thus for this protein we can have a fairly sophisticated estimate of exactly what would be needed to make a functional molecule. At each of the 110 amino acid sites we can determine what substitutions are allowed across the whole spectrum of sequenced proteins. For example, at position 93, the amino acid present may be Phe, Met, Ile, or Leu. Each variety of cytochrome c protein is fully functional, so we can say a functional protein can result with any of four of the twenty amino acids at position 93. A similar calculation for each amino acid position can give us a useful minimal probability of obtaining a cytochrome c from random permutations of amino acids. Careful calculations by Hubert Yockey (1992) demonstrate that with all amino acids present in equimolar amounts and no competing molecules besides stereoisomers, a functional cytochrome c molecule could be obtained in only 2 x 1075 tries. If one accepts Saganís optimistic estimate for the number of amino acids present in his primeval soup of 1044 amino acids, and if we could simultaneously add one new amino acid to each of 1044 growing chains, once each second, proceeding only until failure, only 1023 years would be required to have a 95% probability of obtaining a functional molecule of cytochrome c in this system. That's ten trillion times the generally accepted age of the universe. As it turns out cytochrome c is a very liberal molecule compared to, say, histone H3 protein which is so invariant that only three of 125 amino acids are different between histone H3 of a pea and that of human. To make a single correct histone protein in the same system would require nearly 1060 years at the 95% confidence level, if only alpha linkages were formed and only l-amino acids were present and no competing non-proteinous amino acids were present and if we had a system where such trials could be accomplished. Both of these stories are assuming that we have such a system, and we have already seen that we do not. In short the synthesis of protein or nucleic acid with information cannot happen.
††††††††††† We have reached an impasse. Up to this point we have carefully covered the ground looking for any possible solutions to the origin of life quandary. Even if we spread the probability calculations with all possible functional substitutions for one protein we know the most about, we can see that it is virtually impossible, even under the most unrealistic optimistic conditions. How then can we make a living cell? We canít even make a single functional protein! Either we stop here or we go on to bury the arguments for abiogenic origins deeper. We shall go on.
Origin of Cells:
††††††††††† What is required? Cell is defined as a self-replicating living unit capable of growth, metabolism and other functions associated with life. We will focus on the self-replicating aspect of the cell in order to determine the likelihood that a cell could have arisen by chance. If we can visualize a minimal requirement, we can then ask intelligently the question as to whether such an entity might be capable of self-origination. The requirements are formidable---first we must have the information required for cellular construction, since without information, life and cell construction are not possible. All living cells contain precise specific information on their makeup and division, in the form of DNA. This DNA is a molecular representation of codified information for the processes and structure of life. We can argue without substance about where the information originated, but human experience and cybernetic analysis tell us information comes from an informer, thus necessitating the existence of an information giver. A number of respected scientists including the astronomer Hoyle, the Paleontologist Patterson, the cyberneticist Yockey and others have reached similar conclusions for very different reasons. Nevertheless the belief persists that if you had the right conditions, for the right amount of time, anything might be possible. We will thus analyze this proposition to test its validity.
††††††††††† Let us ask what the minimal requirements for a living cell are. All cells must have a membrane made up, in the simplest cases, of triglycerides or phosphoglyceride lipids associated with specialized proteins that stabilize the membrane and assure its structural integrity. Artificial lipid bi-layers can be seen to form spontaneously into spherical cell-like structures. Thus one might conclude that the presence of phospholipids in the prebiotic sea would seem to assure that the container for cells was present. But the picture is not so simple. Fatty acids, the primary component of all cell membranes, have been exceedingly difficult to produce under abiogenic conditions, even those with reducing atmospheres. Even if such molecules were produced, divalent cations such as Mg++ and Ca++ would combine with the fatty acids, and precipitate them to the sea floor to be incorporated in the Precambrian sediments. Thus even if they had been formed initially, they would be unavailable for membrane formation. These are complex molecules that would certainly not be common under primitive earth conditions. The existence of cellular bounding membranes is thus far from assured. But the problem goes further, since a phospholipid membrane is impermeable to most molecules the cell would need to grow. Membranes in modern cells circumvent this problem by having as integral components very sophisticated proteins that selectively admit wanted molecules. It is of course not conceivable that such proteins were available to the first protocell. Thus the existence of a cellular bounding membrane would hinder the development of a protocell, yet without a membrane there can be no cell. Another complex question. What now?
††††††††††† Let us try another approach---forget the cell, forget the membrane---what would be required as a base minimum just to make a protein molecule. We could imagine proteins smaller than modern proteins, say 100 amino acids long, using less than 20 of the proteinous amino acids, a less than perfect polymerase system, perhaps as few as 100 specific proteins total, maybe even 80. Letís even suppose they could also use non-proteinous amino acids, and that either enantiomer would work. All of these assumptions are ludicrous. We have no starting materials, not even the right ones. We have no idea how we could make a polymer of 100 amino acids under prebiological conditions. These is no possibility that the ridiculously nonstringent conditions could produce a self-replicating system. But since we are playing this game lets make it even worse. Of the 80 proteins we said we needed, lets let the first 60 have any sequence of amino acids at all. Of the remaining 20 proteins, the first has one amino acid specified. The other 99 can be any amino acid. The second has two specified, and so on until the twentieth has twenty amino acids specified. We will let the ocean be two miles deep over the entire earth and the concentration of amino acids 1 molar for each species. We will divide the ocean into one liter increments and consider the feat accomplished when any one liter produces all of the requisite proteins. We will allow the proteins to be made at the rate of a million tries per liter per second. We will assume the same probability for nucleic acids. With all these assumptions made in favor of producing our exceedingly liberal primitive cell, we will achieve the intended result with a 50% probability once in 10186 years.
††††††††††† This figure is of course incomprehensible. To give you an idea of how incomprehensible, I use the following illustration. An ameba starts out at one side of the universe and begins walking towards the other side, say, 100 trillion light years away. He travels at the rate of one meter per billion years. He carries one atom with him. When he reaches the other side, he puts the atom down and starts back. In 10186 years, the ameba will have transported the entire mass of the universe from one side to the other and back a trillion trillion trillion trillion trillion trillion times. That is my definition of impossible. And what resulted from success, if it did occur would not be a living cell or even a promising combination. Spontaneous origin of life on a prebiological earth is IMPOSSIBLE!
Alternatives to Abiogenesis:
††††††††††† What are the alternatives? Several have been proposed:
††††††††††† 1) Origin on another planet. How does this help? We have already investigated the most optimal conditions possible and found them not to be conducive. Putting the process off to another place is an admission of failure.
††††††††††† 2) Biochemical Predestination. An effort to attribute the properties of living systems to the molecules from which they are formed. A popular book by this title in 1969 suggested just such a scenario. This thread has been taken up by complexity theorists and sociobiologists. But ultimately the viability of a model is not dependent upon whether it is an attractive concept, but by whether it is true. There is no evidence that biological precursors are energized to make living cells. One of the authors of the book, Dean Kenyon, is now a creationist.
††††††††††† 3) Creation by an Intelligent Power outside our sphere of investigation. This possibility is best investigated by considering what the alternatives are. We have done that. Certainly one searching for truth cannot arbitrarily exclude this possibility.
††††††††††† In light of these alternatives, the concept of creation becomes exceedingly attractive, not just as an alternative, but as the only reasonable alternative. Only someone unwilling to admit the possibility of a Superior Intelligence would exclude this consideration. This conclusion at least makes sense of the many observations we have considered, explaining the source of the information, the reason for synchronicity of stereoisomers, the nonrandom arrangement of genetic codes, answers the unsolvable puzzle of which came first, proteins or genetic code. Scientists ought to welcome a solution that brings understanding and order out of chaos. Scientists ought to be the first to welcome their Creator!
References
Abelson, P. H. (1966). Chemical
events on the primitive Earth. Proceedings of the National
Brooks, J. & Shaw, G. (1973). Origin and Development of Living Systems.
Cairns-Smith, A. G. (1982). Genetic Takeover and the
Mineral Origin of Life.
Cairns-Smith A. G. (1993). Seven Clues to the Origin of Life: A Scientific
Detective Story.
Day, W. (1984). Genesis on Planet
Earth.
Delbruck, M. (1986). Mind from Matter? An Essay on
Evolutionary Epistemology, eds
G. S. Stent, E. P. Fischer, S. W. Golomb,
D. Presti & H. Seiler.
Gould, S. J., Luria, S. E., and Singer, S. (1981). A View
of Life.
Hulett, H. R. (1969). Limitations on prebiological synthesis. Journal of Theoretical Biology 24, 56-72.
Joyce, G. F. And L. E. Orgel
(1993). Prospects for understanding the origin of the
RNA World. In The RNA World, ed. R. F. Gesteland and J. F. Atkins.
Lazcano A, and S. L. Miller. 1996. The Origin and Early evolution of life: Prebiotic chemistry, the pre-RNA world, and time. Cell 85:793-798.
Ohmoto, H. (1997). Evidence in pre - 2.2 Ga paleosols for the early evolution of atmospheric oxygen and terrestrial biota. Geology 24:1057-1184.
Ohmoto
et al. (2006) "
Pflug, H. D. (1984). Early earth geological record and the origin of life. Naturwisschenschaften 71, 63-8.
Schidlowski, M. (1976). Archean atmosphere and
evolution of the terrestrial oxygen budget. In The
Early History of the Earth, ed. B. F. Windley.
Schopf, J. W. (1972). Precambrian
Paleobiology. In Exobiology,
ed. C. Ponnamperuma.
Shapiro, R. (1986). Doubt and uncertainty; Bubbles,
ripples and mud. In Origins, A Skepticís Guide to the Creation of Life
on Earth, Chapter 8, pp. 190-224.
Shapiro, R. (1986). Prebiotic ribose synthesis: a critical analysis. Origins of Life 18, 71-85.
Sillen, L. G. (1965). Oxidation state of Earthís ocean and atmosphere, Arkiv for Kemi 24, 431- 56.
Usher, D.A. and McHale, A.H. (1976). Hydrolytic stability of helical RNA: a selective advantage for the natural 3',5'-bond. Proc. Nat. Acad. Sci. U.S.A. 73: 2174-2179.
Walker, J. C. G. (1976). Implications for atmospheric evolution of the
inhomogeneous accretion model of the origin of the Earth. In
The early History of the Earth, ed. B. F. Windley.
Walker, J. C. G. (1977). Evolution of the atmosphere.
Woese, C. R. (1979). A proposal concerning the origin of life on the planet Earth. Journal of Molecular Biology 13, 95-101.
Yockey, H. P. (1992). Information
theory and molecular biology.
Yuasa, S., Flory, D., Basile & Oro, J. (1984). Abiotic synthesis of purines and other heterocyclic compounds by the action of electrical discharges. Journal of Molecular Biology 21, 76-80.