"A New Method for Testing Models of Prebiotic Peptide Assembly"'
© 2000, Melanie BENGTSON and Eric D. EDSTROM*
Department of Chemistry, University of Montana, Missoula, MT 59812
I. Introduction
The modern theory of chemical evolution is based on the assumption that on a primitive earth a mixture of simple chemicals assembled into more complex molecular systems, from which, eventually came the first functioning cell(s). In this extremely complicated series of transformations, several key transitions must be contemplated. Two areas which present particular difficulties for the theory is the origin of enantio-enriched biomolecules and the polymerization of simple monomers into information rich networks. At the molecular level, L-amino acids and D-sugars serve as the fundamental building blocks for two of the most important biological polymer networks necessary for all forms of life. The former give rise to a vast array of protein structures whereas the common D-ribose framework, coupled with the five well known heterocyclic bases and linking phosphate groups, generates the core nucleic acid structure characteristic of DNA and RNA. Within this structural motif the genetic code is the primary source for all information essential for the existence of life.
The origin of biological chirality and the sequence information residing the genetic code remain unsolved mysteries in science. Ever since the pioneering discovery of molecular dissymmetry by Pasteur in 1848, numerous theories have arisen to explain biological enantiospecificity, however, no conclusive experimental evidence has been provided to support any hypothesis offered to date1 The crux of the debate lies in the question of how and when did the enantiospecific characteristics of life originate--before, during, or after the origin of life on earth? In recent conferences on the topic, an emerging view among researchers is that biochemical processes have played a greater role than abiotic physical processes.
In regard to the construction of information rich (coded) sequences reticent in proteins and genetic material, their is a haunting lack of experimental evidence provided in the literature. This appears despite hundreds of published works spanning nearly four decades of work focused on prebiotic models for peptide and, to a lesser extent, nucleic acid synthesis. It is the aim of the short review to examine lines of evidence given from prebiotic reaction simulations, leading to the formation of simple peptides from amino acids, with regard to these two aspects. In order to more clearly discern the dilemma of which came first, enantioselection or life? the importance of sequence information needs to be brought into the discussion. A new and discriminating model for testing the validity of all the models of prebiotic peptide assembly will be proposed which addresses these two critical issues simultaneously.
II. Which Came First?
A central dilemma facing all origin of life theories is the question of which came first, enantio-enriched biomolecules or life? This much debated topic has been the focus of a recent conference2a and is germane to topics presented in the current symposium.2b The two major perspectives that have developed over the years are summarized below.
In the first view, recognition is given to the fact that all life processes that we observe today depend upon chemical-handedness, e.g., genetic replication, protein biosynthesis, substrate binding, enzyme catalysis, and antigen-antibody interactions. This requires that'a mechanism or process providing a source(s) enantiopure molecules had to be in place prior to the development of the first living organism. In an effort to account for this mechanism, two classes of factors that are able to break symmetry in chemical systems have been argued; effects of chiral fields (both local and global) and spontaneous symmetry breaking.1 Among physicists and theoretical chemists enantioenriched molecules are seen to be an inevitable consequence of universal fundamental physical processes which took place either directly on earth or in extraterrestrial environments. These are centered on spontaneous symmetry breaking due to weak nuclear interactions between elementary particles attributed to [~-decay and weak neutral currents. The major criticism for this model is the exceedingly small parity violation energy difference between enantiomeric biomolecules which resides in the range of 10-15 to 10-17 kT.
In the other model chiral fields based on circularly polarized light, electrical, magnetic, or gravitational forces are invoked. Extraterrestrial speculations are popular due to problems with entropic phenomenon on a earthly setting;. However, the mode of delivery and preservation of enantioenriched materials to the earth's surface and their incorporation into living systems remains an unsolved mystery.
Perhaps the most vexing question facing these explanations based on abiotic processes, lies not on whether the accumulation of pools of enantioenriched organic compounds can occur,3 but rather the need to address to the problem of transferring asymmetry from the chemical level to the bioorganic world. This critical aspect has not been addressed either in a theoretical or empirical manner. It clear is that the basic relationship between asymmetry at the level of simple organic compounds that may have arisen during the course of chemical evolution and the chiral specificity of the biological macromolecules intrinsic to living systems is not known.4
In strong opposition to various abiotic physical models is a biotic (or life first) model advanced primarily by chemists. The principle argumentin this group is based on the known chemical instability of alleged prebiotic molecules under geochemical reaction conditions, the most noted example being D-ribose, a key constituent in RNA.5 One proposal advanced suggests that the first self-replicating molecules present in a pre-RNA world were made up of achiral constituents. Peptide nucleic acids (PNA) were offered as possible candidates for early carriers of genetic information.6 In another scenario isotactic polymers (either all L-or D-based handedness) would be initially formed in equal amounts and by some unknown chance process an evolutionary advantage was selected for one enantiomeric form over the other. Thus the origin biological enantiospecificity would have been closely associated with the origin of life. In this view chiral discrimination in biological reactions are not seen to be important to life's primitive structures and processes. As a consequence, a vastly different metabolism would be required in early earth history. This necessitates an explanation for the transition to the enatiospecific metabolism common to all life forms currently known. No adequate or convincing theoretical models or empirical evidence for such a transformation has been forthcoming. In contrast, evidence exists that suggests that certain enzyme function, i.e., long-range proton transfer is critically dependant on L-configurations for every peptide residue.7
In common with the physical models, the biotic model does not provide a mechanism for explaining the origin of sequence information with any conceivable type of primitive metabolism. Furthermore, the prebiotic synthesis of pre-RNA frameworks can not be addressed due to a lack of certainty regarding the actual nature of such an assembly.
III. Models for Prebiotic Protein Synthesis
Models for the prebiotic synthesis of proteins have been considered for nearly four decades. A brief summary of the developments over this time period is needed in order to attempt to understand the current state of affairs in this area of chemical evolutionary theory. Following this historical .treatment, some effort will be spent to examine more closely the major findings from three of the most recognized models for prebiotic peptide assembly within the context of the evolution of biological enantioselection and the origin of sequence information. The focus will lean towards protocols that use unactivated amino acid precursors in contrast with those that utilize various types of activating agents or activated precursors? This is due to the conceptual and probability difficulties involved in introducing additional reagents or prepared intermediates in the random, unguided processes required by chemical evolutionary theory.
Early efforts by Fox and coworkers focused on heating mixtures of amino acids in the solid state at high temperatures (130-180oC) and gave rise to proteinoid materials with a variety of interesting properties.9a Although high molecular weights could be obtained with an excess of acidic or basic amino acids present, the exact composition of these materials in terms sequence information was never well characterized. Furthermore, heating mixtures of selected L-amino acids gave rise to a polycondensate which was only 50% peptidic. This fraction was found to be racemized and the peptide linkages are ambiguous due to nearly random linking at the a, b, and g carboxylate functions of the dicarboxylic amino acids. This model has lost favor due to the necessity of having high surface temperatures on the face of the primitive earth which would be destructive to other types of prebiotic compounds. A later study showed that heating aspartic or glutamic acid with a mixture of 16 proteinoid amino acids under 100oC gave rise to an uncharacterized polymeric material.9b
Alternative models based on reactions over clay minerals surfaces and the solid phase were initially developed from the late 1960's through the late 1970's. In this time numerous difficulties (vide infra) in the existing models were recognized despite considerable experimental efforts. The likelihood of a protein first world on the earlyearth began to enter into considerable doubt, as a result. This lead, in part, to a major revival of the RNA-world hypothesis that was prompted by the discovery of catalytic RNA in 1982.10 In recent years this initial exuberance in this movement has dampened considerably due to severe difficulties that arose from subsequent experimental studies. Namely, the inability to clearly define a viable route to nucleoside monomers and enantiomeric cross-inhibition during assembly of nucleotide monomers of mixed chirality.11 As well, numerous difficulties in explaining the chemical origin of the RNA backbone constituents, i.e., the base portion,12a the ribose portion,12b and the type of phosphorylation process13 were realized and have come under sharp criticism.
In view of these difficulties, the advent of the pre-RNA world hypothesis has become prevelant in-the past decade.14 More recently, a reemergence of published studies involving prebiotic models for peptide synthesis has arisen after a period of lessened interest during the wake of the RNA-first revolution15,18 This can be seen as a necessity for some when considering proposed pre-RNA molecules such as PNA's whose backbone is comprised of two types of amide (peptide like) bonds.6 Thus, a reexamination of the key experimental findings from earlier studies coupled with the results for more resent efforts is in order.
A. Clay Mineral Surfaces.
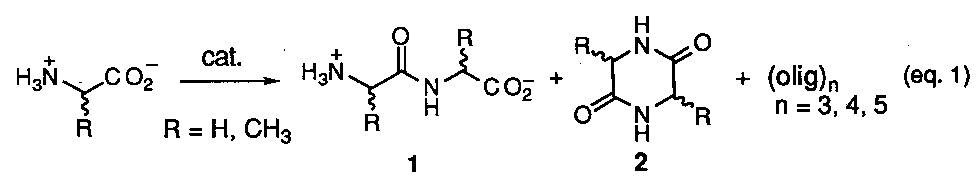
Clay mineral surfaces have attracted interest as catalysts in prebiotic reaction scenarios due to their widespread abundance over the earth's surface and have served as the basis for many early studies.16 The most realistic reaction scheme involving the condensation of unactivated amino acids is summarized as shown below (eq. 1) The typical experiment procedure involved exposure of a single amino acid to various clay minerals or silicates and then subjection to aqueous wet-dry cycles brought about by temperature variations in the range of 25 - 100o C or less. This is thought to mimic alternating wet-dry cycles on the early earth surface with corresponding heating and cooling periods. The varieties of materials examined are based on those which are common to the earth's surface today and have included montmorillonites, kaolinites, bentonites, silica gel, and alumina. After a period of cycles the crude reaction mixtures where analyzed for oligomeric products using chromatographic techniques. In all cases the total combined yield of oligomers varied in the range of 0.02 to 3.8%. The major products formed were the dimer 1 and the cyclic anhydride 2 (a diketopiperazine) with only traces of trimer up to pentamers being detected. The scant yields of oligopeptides was attributed to cyclic anhydride formation and hydrolysis of the longer peptide chains. In nearly all cases only glycine (R = H) was used as the amino acid. Unfortunately these efforts provided no criteria to evaluate the role of enantioselection or sequence information in prebiotic peptide synthesis. In one case a claim was reported that kaolinite catalyzes the polymerization of D- and L-aspartic acid at different rates, but this was soon refuted.17

It hasn't been until the recent work of Bujdak and coworkers that use of a mixture of two different amino acids has been seriously addressed within this model.18 Combinations of alanine (ala) + glycine (gly), ala + diglycine (gly2), and ala + gly cyclic anhydride [cyc-(ala-gly)] over silica, alumina, and montmorillonite were studied. Overall, complicated reaction mixtures resulted in the formation of all possible varieties of dimeric products, i.e., ala-ala, gly-gly, ala-gly, gly-ala, cyc-ala2, cyc-gly2, and cyc-(ala-gly), the amounts of which, varied depending upon the catalyst used and the reaction conditions. Most significantly was the report that the mixed cyclic anhydride, cyc-(ala-gly), can be converted back by hydrolysis into two different dipeptides, ala-gly and gly-ala, thus demonstrating a mechanism for the scrambling of sequence information. This fact coupled with the previously known preponderance for cyclic anhydride formation, and hydrolysis of longer peptides casts considerable doubt on whether peptide formation over clay mineral surfaces can be considered a viable prebiotic source of oligopeptides having defined sequence information.
B. Studies Involving Activated Amino Acids
Reactions involving.various types of activated amino acids or activating reagents have yielded sgme data related to the generation of enantiopure homopeptides although no peptides having sequence information have been reported. Two concerns regarding the use of activating agents from a prebiotic reaction perspective is the introduction ora more complicated design criteria19 and the hydrolytic instability of activating reagents and their derived intermediates, although in one study short peptide segments were obtained under aqueous conditions via the intermediacy of N-imidazoylcarbonyl amino acids.20a To avoid the hydrolysis issue, a number of studies have been conducted under solid phase conditions. A representative study is shown below (eq. 2).20b Here an evaporated mixture of glycine, ATP, and imidazole was heated at 65-90o C up to 48 hrs in the presence and absence of metal salts. At best, oligomers up to the decamer level were detected, however, significant amounts of. cyclic anhydride 3 plus undetermined side-products were formed. Most problematic was the fact that these nonfunctional materials formed at the expense of the linear peptides and increased over time. Recently, separate reactions of three different amino acids in the presence of carbonyldiimidazole and cationic micelles was found to improve the yield of oligomers up to heptamer length under more dilute aqueous reaction conditions. The all L-configuration of the residues in the peptide chain was retained.20c
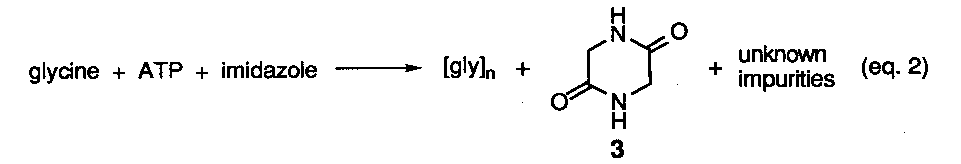
C. A New Thermal Model
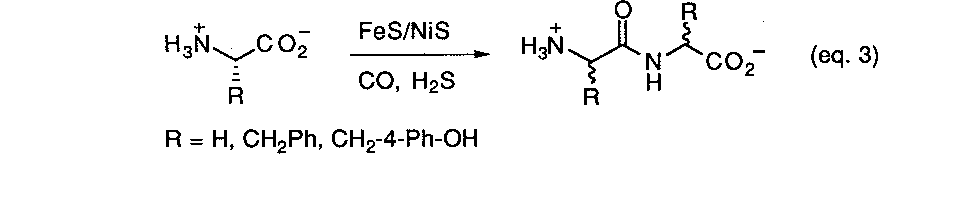
As summarized earlier, some of the first models for prebiotic peptide synthesis developed by Fox, et. al.9a involved the simple thermal heating of mixtures of simple amino acids. The basic chemical driving force leading to the formation of the peptide bond is the thermal expulsion of water. In the past decade much interest has. arisen over the discovery of a multitude of unique thermophilic organisms living in geothermal areas. Prebiotic reaction scenarios have naturally turned to modeling volcanic or hydrothermal settings as a possible setting for the origin of life. Recently Wächtershäuser reported21a a new scheme for activating amino acids for peptide synthesis which claims to avoid many of the problems associated with earlier approaches, namely, the extensive formation of cyclic anhydrides and the requirement for introducing activating reagents which have no plausible prebiotic source. The method involves the use of coprecipitated NiS/FeS in conjunction with a thiol source as a catalyst and condensing agent at 100o C under anaerobic, aqueous conditions (eq. 3). It is claimed that this combination is a more realistic prebiotic reaction process and closely simulates the environment that would be found in a volcanic zone or an oceanic hydrothermal vent. Using three different amino acids (glycine, phenylalanine, and tyrosine) mostly dipeptides were formed in small amounts with traces of tripeptides being detected. Thee lack of formation of longer peptides was attributed to competing hydrolysis reactions under the reaction conditions. Such a scrambling mechanism would also prevent the accumulation of meaningful sequence information. Furthermore, experiments with L-phe and L-tyr resulted in dipeptides that were extensively racemized after a period of 4 days. The loss stereochemical information in this way presents a problem for any theory which requires the existence of enantioenrichment prior to the emergence of life. On the other hand this is claimed to present no difficulties for a chemoautotropic origin of life model.21b No demonstrated basis for this claim was presented however.
IV. A New Basis for Testing Existing Theories
In spite of a multitude of theories and experimental studies, the formation of peptides, coupled with the appearance of all L-configurations of the individual amino acid residues, remains an unsolved mystery in the origin of life. In contemplating this state of affairs we felt that it would highly desirable to develop some simple experimental tests to validate existing models of prebiotic peptide assembly in an effort to reduce the number plausible scenarios. It was also desired to incorporate a test that would examine these two aspects simultaneously so as to make the results more definitive in nature. With this criteria in mind the following model was formulated and is considered to be appropriate for testing all prebiotic peptide synthetic schemes. Rather than attempt to assemble peptides, we reasoned to incorporate a method commonly used in chemical reaction product studies where new centers of chirality are formed. To establish whether a reaction product stereochemistry for a given reaction is the result of either kinetic or thermodynamic (equilibration) control, the reaction product is reexposed to the reaction conditions and assayed to determine whether a change in the ratio of newly formed stereoisomers is observed. Such a test is particularly relevant to cases where the process of epimerization is likely as the case where the stereocenter is adjacent to a carboxyl group. Numerous studies have documented the process of racemization in amino acids and simple peptides.22 Thus, the model is presented as follows.
1. Do enantioenriched, i. e., all L-dipeptides, tripeptides, etc. maintain stereochemical and sequence integrity during prebiotic reaction conditions?
On this Basis the Following Conclusions can be Derived
1. If stereochemical and sequence information is retained, then the simulation would stand as a valid prebiotic reaction.
2. Contrariwise, if stereochemical or sequence information is lost over measurable periods of time, then the likelihood of the simulation serving as a realistic prebiotic reaction would be cast into greater doubt.
3. Alternative scenarios for explaining the formation of information-rich molecular sequences must be considered.
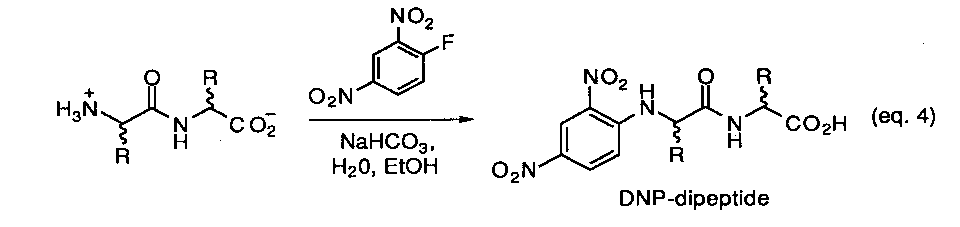
The experimental approach that is under development for this model is based on using a chiral-phase HPLC method developed to analyze dipeptide derivatives by Pirkle and coworkers.23 We are currently preparing UV-labeled derivatives for each of the stereoisomers (L/L, D/D, L/D, D/L) within a set of selected homo- and heterodipeptides. As a typical example, the classical Sanger method for making amino acid N-dirdtrophenyl derivatives offers a convenient and well defined possibility (eq.4) Once a suitable chromatographic assay is established so that each isomer can be separately detected, the appropriate L,L-dipeptide will then be exposed to various types of prebiotic reaction conditions and monitored as a function of time and temperature. Aliquotes containing the original dipeptides along with any other possible reaction products will be derivatized as either the N-dinitrobenzoyl- or N-dinitrophenyl compounds and directly subject to HPLC analysis. It is expected that the results of this study will provide a simple and effective method to survey a number of prebiotic processes in short order.
V. Conclusions
On the basis of this brief commentary, certain conclusions can be reasoned and placed on firm ground. Within the scope of the theory of chemical evolution that deals with the prebiotic synthesis of peptides, no established method is available to explain the complexity that is reticent in even the simplest peptide hormone known today in terms of sequence and stereochemical information. To date no serious experimental effort has focused on these issues by incorporating chiral amino acids and mixtures of amino acids into their prebiotic reaction schemes. The anticipated complexity of the resulting product mixtures using existing models likely forebodes such an adventure. As an approach to this impasse, a simple model is provided which allows for the testing of various prebiotic models by evaluating the stereochemical and sequence stability of preformed di- and tripeptides under prebiotic reaction conditions.
VI. References
1. Mason, S. Chem. Soc. Rev. 1988, 17, 347.
2. a) Bada, J. L. Nature 1995, 374, 594. Cohen, J. Science 1995, 267, 1265. b) Symposium
on Biological Homochirality, Serramazzoni (Modena) Italy, September 6-12, 1998. 3. Siegel, J. Chirality 1998, 10, 24.
4. Avetisov, V.; Goldanskii, V. Proc. Natl. Acad. Sci. USA 1996, 93, 11435. 5. Miller, S. Proc. Natl. Acad. Sci. 1995, 92, 8158. 6. Nielsen, P.; Orgel, L. Nature, 1995, 376, 578. 7. Fisun; Savin, Biosystems 1992, 27, 129.
8. For a discussion of various activation schemes, see: Lohrmann, R.; Orgel. L. E.
Nature 1973, 244, 418.
9. a) Fox, S. W.; Dose, K. D. Molecular Evolution and the Origin of Life Marcel Dekker,
New York, 1977. b.) Rohlfing, D. L..Science 197.6, 193, 68.
10. For a comprehesive coverage of this area, see..Gesteland, R.F.; Atkins, F. Eds. The
RNA-World; Cold Spring Harbor Laboratory Press; Plainview, NY, 1993.
11. Joyce, G. Nature 1989, 338, 217.
12. a) Shapiro, R. Origins of Life and Evol. Of the Biosphere 1995, 25, $3. b) Shapiro, R.
Origins of Life and Evol. Of the Biosphere 1988, 18, 71.
13. Orgel, L. Science 1971, 171, 490.
14. Piccarelli, J. Nature 1995, 376, 548.
15. Brack, A. Pure & Appl. Chem. 1993, 65, 1143.
16. Lahav, N.; White, D. H.; Chang, S. Science 1978, 201, 67.
17. Degans, E. T.; Matheja, J.; Jackson, T. A. Nature 1970, 227, 492. McCullough, J. J. J.
Mol. Evol. 1974, 3, 57.
18. Bujdak, J.; Eder, A.; Yongyai, Y.; Faybikova'. K.; Rode, B. M. J. Inorg. Biochem. 1996,
61, 69. Bujdak, J.; Rode, B. M. J. Mol. Evol. 1997, 45, 457.
19. For a summary of amino acid activation schemes in aqueous environments, see: Orgel, L. J. Mol. Evol. 1989, 29, 465. Paecht-Horowitz, M.; Eirich, F. R. Origins of Life 1988, 18, 359. 20. a) Ehler, K. W.; Orgel, L. Biochem. Biophys. Acta 1976, 434, 233. b) Sawai, H.; Lohrmann, R.; Orgel, L. J. Mol. Evol. 1975, 6, 165. c) Bohler, C.; Hill, A. R.; Orgel, L. E. Origins of Life and Evol. Of the Biosphere 1996, 26, 2.
21. a) Huber, C.; Wächtershäuser, G. Science 1998, 281, 670. b) Wächtershäuser, G. Proc. Natl. Acad. Sci. 1990, 87, 200.
22. Schroeder, R. A.; Bada, J. L. Earth-Science Rev. 1976, 12, 347.
23. Pirkle, W.; J. Chromatography 1987, 398, 203.