ORIGIN OF LIFE:
A LOOK AT LATE 20TH-CENTURY THINKING*
Ó
2004 George T. Javor, Ph.D.Among numerous ideas in currency four or five thousand years ago about the origin of life was one that is still held dearly by millions. It is summarized in the fourth commandment: "For in six days the Lord made heaven and earth, the sea, and all that in them is, and rested on the seventh day..."(1).
Much more recent is a radically different concept of origins which derives the present universe from a hypothetical "big bang" and its evolutionary aftermath. Accordingly, life originated on Earth by random interaction between matter and energy.
Never before has the phenomenon of life been better understood. This is due to intense research effort by tens of thousands of scientists and their often spectacular discoveries over the past 50 to 80 years. The functions of about one-third of all proteins manufactured by the simple cell Escherichia coli are now known, and the total elucidation of the structure of this cell is foreseeable (2).
We are also learning more about structures and workings of other more complex living systems. The recent development of automated DNA sequencing has prompted suggestions that a multi-billion dollar effort be organized to determine the complete nucleotide sequence of the human genome.
Cells are the smallest living independent entities, and nothing less than a cell deserves the adjective "alive." Cells range in complexity from the simple bacterium, such as the common colon organism Escherichia coli, to highly differentiated cells of our nervous system. Numerous features common to all cells are understood by creationists to signify a common designer, but are explained by evolutionists in terms of a common ancestry.
Common constituents of living matter
All cells have similar components. By weight they are 60-70% water, 25-35% biopolymers, and about 5% small organic compounds and minerals. These cellular ingredients (with the exception of water and minerals) are unique in several ways and cannot be found in nature except as parts of living or once-living matter.
Our inanimate environment comprises substances made from comparatively simple molecules containing a limited number of atoms. These molecules are rich in oxygen atoms, resistant to heat, and generally stable under a variety of conditions. In contrast to these simple molecules, biological polymers (which constitute most of living matter after water is removed ) -- proteins, nucleic acids, polysaccharides and lipids- are molecules made from thousands of atoms. They are rich in carbon and hydrogen atoms and are definitely unstable in the presence of heat and oxygen. Researchers working with proteins, for example, must always be careful not to stir a protein solution too vigorously, and to keep it on ice as long as possible, so as to prevent unraveling their intricate structures.
Protein molecules and nucleic acids are informational macromolecules, i.e., their structures harbor biological information. The gigantic molecules of proteins and nucleic acids are made by linking hundreds (or thousands) of a small number of "building block" molecules: amino acids for proteins and nucleotides for nucleic acids. Biological information resides in the particular sequence in which building blocks are linked.
When letters of the alphabet are linked in particular sequences, meaningful words are created. Likewise, the information content of proteins and nucleic acids depends initially on the order in which their building block components are connected.
The true meaning of biological information contained within the structures of biopolymers is evident only in the context of the entire living cell, because the phenomenon of life depends on harmonious interactions of thousands of kinds of protein and nucleic acid molecules. If biopolymers are like words, then the living cell is like an extensive monograph.
Biopolymers mixed in test tubes do not yield living matter
When all biopolymers are removed from a cell and put in a test-tube with all the other ingredients normally found in cells (small organic molecules and minerals) in just the right proportions, nothing happens.
The living cell is more than a collection of biologically active molecules. However, the extra quality is not, as many think, a mysterious life force which departs upon death. This may be demonstrated rather dramatically by freeze-dried bacteria.
If a liquid culture of single-celled organisms is frozen rapidly and placed under vacuum, cellular water in the form of ice gently leaves the cells through sublimation, leaving behind cells as waterless powder. The organisms are in a state of suspended animation, neither alive nor dead. They can remain in this state indefinitely, so long as they are kept dry. If the cells are placed in water along with suitable nutrients, they once again continue living. Therefore, in this instance "life" was manipulated simply by adding or removing water.
Why living matter is more than the sum of its ingredients
In a living cell the thousands of chemical transformations that are necessary for life to occur must be confined to a comparatively small space. This makes the products of one reaction available as starting materials for the next reaction along the necessary biochemical metabolic pathways. Moreover, the ingredients of cells are frequently assigned spatially, some in the nuclear region, others near the cell envelope. Without cellular morphology, these components have no meaningful tasks.
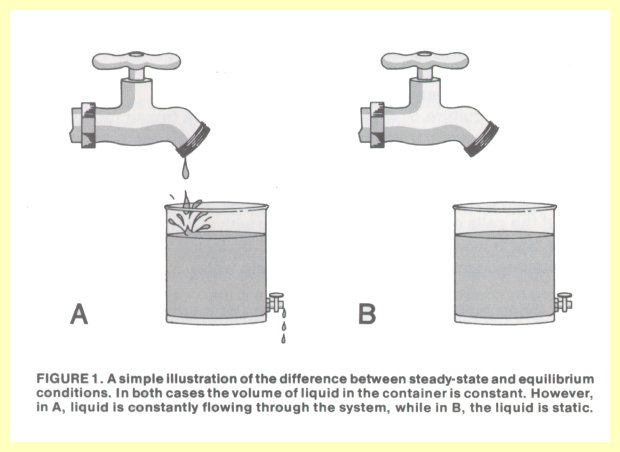
The process of life is dynamic, involving the biosynthesis of new substances, degradation of old ones, pumping in fresh food supplies and secreting waste products, as hundreds of chemical changes take place simultaneously every second. A most important property of a living cell which makes it more than just the sum of its ingredients is that the totality of its chemical transformations is not in equilibrium.
A chemical change is the rearrangement of atoms making up various molecules. Such a change may be represented as: A + B
Û C + D, where substances A and B interact and form products C and D. After this chemical change runs its course, a certain amount of all four substances will be present. The ratio at equilibrium of (C* D) to (A* B) is an unchanging (constant) number. At that point the reaction is incapable of any further chemical transformation. If all these chemical changes reach equilibrium, the cell dies.Essentially all chemical reactions in a cell are facilitated by biological catalysts called enzymes. These agents tend to push reactions rapidly toward equilibrium, even though total equilibrium would be fatal to the cell. However, since chemical reactions in the cell are interconnected, the end products of one chemical transformation become the starting material for the next, and thus equilibrium is never reached. As the products are further utilized, more starting materials are manufactured, resulting in constant intracellular concentration of metabolic intermediates. This is called a steady state, non-equilibrium system, because the amounts of metabolic intermediates are relatively unchanging within the cell, and the total system is not at equilibrium. Such is only possible in live, intact cells. If a cell is physically disrupted or if it dies, the steady state changes into equilibrium. Figure 1 illustrates in a simple way the contrast between steady state and equilibrium conditions.

This situation can actually be approximated in the laboratory, by poking holes in the membranes of live cells (so they will lose their ability to concentrate nutrients from their environment) and allowing the internal reactions to go to equilibrium. Such cells are now dead, and even if the holes of their membranes were repaired, they will not come back to life. For life to recur, non-equilibrium conditions would have to be established by the selective removal of key metabolite molecules from the cell. When the strategic reactions are once again restored to non-equilibrium, the system as a whole will be driven toward a steady state.
Manipulations involving the removal of a few small molecules from a cell containing many other molecules is beyond our present and most likely future capabilities. Such a capacity is tantamount to being able to reverse death to life on the cellular level.
Attempts to discover the origin of life
The earliest historical records indicate that man has recognized the qualitative difference between living and non-living matter, and since then there never has been a shortage of theories to explain the presence of life on Earth. Yet the origin of life remains one of the greatest challenges to naturalistic interpretations. According to Nobel laureate Max Delbruck, "... there has been an immense conceptual gap between all present-day life and no life," and the "how" of the transition of earth from no life to life is "perhaps the fundamental question of biology" (3).
Nevertheless, the immense conceptual gap between life and non-life is neither recognized nor admitted by many evolutionary theorists. A 1978 review entitled "Chemical evolution and the origin of life" begins with these words: "Perhaps the most striking aspect of the evolution of life on earth is that it happened so fast' (4). More recently, the first chapter of a college textbook on the molecular biology of the cell contains this summary statement: "Living cells probably arose on earth by the spontaneous aggregation of molecules about 3.5 billion years ago" (5).
Regardless of their degree of optimism or enthusiasm, evolutionary theorists are forced to propose explanations for the spontaneous generation of life from non-living matter. In order for biological evolution to begin, some starting material is necessary. This need is met by the postulates of chemical evolution.
When the outlines of modern theories of chemical evolution (the natural processes on a "pre-biotic earth" which gave rise to the first living matter) were formulated by A.I. Oparin and J.B.S. Haldane in the 1920s (6), very little was known about the biochemical intricacies of living matter. Consequently, there was plenty of freedom to postulate mechanistic processes by which organisms could come into existence.
Modern theories of chemical evolution found in current monographs and textbooks developed over a span of approximately 60 years. They suggest that early Earth was covered largely with a warm, slightly alkaline ocean. Though rich in carbon monoxide, carbon dioxide, ammonia, methane, hydrogen, and nitrogen, the atmosphere definitely did not contain atomic or molecular oxygen. Ultraviolet light from the sun, geothermal energy from volcanoes, shock waves from thunder, and cosmic radiation acted upon gases of the primitive atmosphere causing the formation of biomonomers such as amino acids, sugars, purines, pyrimidines, and fatty acids. These substances polymerized to form the prototypes of more recent proteins, nucleic acids and cell membranes. In time they coalesced to form the first proto-cell, a collection of polymers enclosed in a membrane. Eventually these proto-cells became increasingly complex, until the first true living cell was born.
Laboratory simulations of chemical evolution
The year 1953 was a banner year for chemical evolution. Stanley Miller, a graduate student working with Nobel prize winner Dr. Harold Urey, published his experiments on the synthesis of amino acids in a simulated primitive-earth environment.
He built a glass apparatus, in which circulating ammonia, methane, hydrogen and water vapor were exposed to electrical spark discharges for one week. Molecules forming in the vapor phase were trapped in water and analyzed. Among the 35 diverse substances identified, 9 were amino acids, almost half of the 20 different kinds found in proteins (7)!
Miller's paper signaled an onslaught of experiments by numerous investigators who varied the starting materials, the source of energy and other experimental parameters. Their efforts yielded 19 of the 20 amino acids, all 5 nitrogenous bases which are crucial to nucleic-acid formation, and a number of important sugars as well (8).
These results serve as a pillar on which chemical evolutionists build their theoretical edifices. Apparently it is indeed possible to envision hypothetical situations where at least the most important metabolic biomonomers may come into existence.
The evolutionary scenario requires the continual accumulation of biomonomers in the primordial ocean until it becomes an "organic soup." The next necessary step on the chemical evolutionary ladder is to link biomonomers into polymers, especially proteins and nucleic acids. This involves the removal of a molecule of water from two biomonomers in order to form a chemical bond between them.
One of the postulates proposed for polymerization assumes that high concentrations of various amino acids accumulated at the rim of volcanoes, where the high temperatures drove off the water molecules, leaving proteins behind. Sidney Fox, the chief proponent of this theory, demonstrated that mixtures of amino acids heated at 200°C for 6 or 7 hours indeed formed protein-life polymers which he called "protenoids." These polymers show weak catalytic activities partially resembling enzymes. When protenoids cool, they form "microspheres" supposedly resembling primitive cells morphologically (9). These structures can "grow" under favorable conditions and "divide" by budding. Interesting as these experiments are, their results reveal serious deficiencies when they are used to support a scenario for chemical evolution.
Deficiencies of laboratory simulations of chemical evolution
The success of the Miller-Urey type experiments depends on the types of gases introduced into the experimental systems. Early models of chemical evolution assumed a primordial atmosphere rich in methane, ammonia and molecular hydrogen, and these gases were used with considerable success. More recent models of the early Earth atmosphere, based on data from numerous space-probes, see the primordial atmosphere resulting mainly from the release of volatile materials trapped by solid particles during the formation of the planet. Thus the composition of an early atmosphere would have resembled the contents of present-day volcanic fumes. These are rich in carbon dioxide and water and have minor amounts of nitrogen, hydrogen sulfide and sulfur dioxide. Pre-biotic simulation experiments using gas mixtures of nitrogen, carbon dioxide and water vapors produced mostly ammonia and nitric acid in the hands of one investigator and formaldehyde in another laboratory (10).
Whatever the composition of the primordial atmosphere may have been, evolutionary theorists agree that it could not have contained atomic or molecular oxygen. All postulated processes of chemical evolution would cease in the presence of oxygen, for oxygen would quickly react with organic compounds formed in the atmosphere, oxidizing them to carbon dioxide and formic acid.
Our present-day atmosphere contains 20% oxygen. A small portion of this gas is converted to the ozone layer of the upper atmosphere which shields us from high-energy ultraviolet radiation of the sun. A primordial earth, covered with an oxygenless atmosphere, would have been subject to the sterilizing effect of ultraviolet radiation. If, on the other hand, there was a primordial ozone shield, then oxygen also had to be present at concentrations of at least 1-1 0% of the current amount.
A potentially important source of pre-biotic oxygen could have been the photo-dissociation of water by ultraviolet rays. Calculations of theoretical levels of oxygen in a primordial atmosphere range from essentially nil to 25% of present levels (11). Support for high rate of oxygen production by dissociation of water vapors comes from data collected during the Apollo 16 mission, where pictures of Earth were taken from the moon, using ultraviolet sensitive films. These pictures showed that a gigantic cloud of hydrogen, extending 40,000 miles into space, surrounded the earth. The source of this hydrogen could only be water vapor, bombarded by high-energy ultraviolet rays above the ozone layer.
Scientists have examined uranium and iron-containing minerals from the earliest available sediments, hoping to learn whether the early atmosphere was oxidizing or reducing. The results were equivocal. We now believe that the existence of reduced minerals in sediments does not necessarily signify the existence of a reducing atmosphere and an oxidizing atmosphere does not always produce oxidized minerals. The relationship between a sediment and its environment cannot be established unless the actual rates of oxidation or reduction are known.
Two further observations should be made about the significance of the Miller-Urey type organic-soup-producing experiments. First, a consideration of yields. Even with the removal of products during experimentation by the use of traps, pre-biotic simulation experiments generate fairly small amounts of usable products. Assuming no destruction of molecules in the atmosphere, optimistic estimates ranged as high as 0.001 M concentration in the primitive ocean. However, when 'the destructive effect of ultraviolet radiation on amino acids is taken into account, the upper limit has been given at one ten millionth molar in the primitive sea, which happens to be the actual concentration of amino acids in the North Atlantic Ocean (12)!
Such Iow concentrations of biomonomers would have been inadequate to polymerize into macromolecules. Though it has been suggested that chemical evolution could have proceeded in smaller pools where the precursor substances would have been concentrated, there is no geologic evidence for large deposits of organic substances. Moreover, if concentration had occurred, undesirable impurities likely would also accumulate and interfere with polymerization, the next step in chemical evolution.
The second observation is that synthetic reactions outside a cell produce equal amounts of optical isomers of amino acids and sugars. Therefore the primordial ocean would have contained a racemic mixture of biomonomers. Since known biopolymers exclusively utilize only one of the two or more possible isomers in the case of sugars and amino acids, it is totally incomprehensible how such an arrangement could develop from a 50-50 mixture of optical isomers.
Thus it is highly unlikely that chemical evolution could have taken place by the organic-soup mechanism. Among the factors against this mechanism are the great likelihood of substantial oxygen content in the primitive atmosphere and the small yields of biologically significant substances which would be present as equal amounts of optical isomers.
The "volcanic rim" approach of Sidney Fox assumes a primordial earth covered with an organic soup. It addresses the next difficulty--the polymerization of biomonomers- by splitting out the water in an aqueous environment, which, in terms of thermodynamics, is essentially impossible! However, by postulating a heat source, he dries up the environment and succeeds in the polymerization. But Fox pays for his success dearly.
The resulting protenoids have only a superficial resemblance to true proteins, in that the resulting peptide bonds are predominantly of the beta, gamma and epsilon variety, rather than the naturally occurring alpha bonds. The amino acid sequences are generated entirely by random means, and there is no mechanism to ensure any reproducibility. If by chance a biologically useful molecule is formed, how will its subsequent production be ensured?
When protenoids cool, they form microspheres which, according to Fox, grow and divide. True growth, however, requires numerous metabolic steps and incorporation of small molecules into the polymer structure of the cell. In Fox's experiment, "growth" results from the physical attraction of opposite charges, and "budding" refers to the breaking up of microspheres due to changes in acidity or heat.
Since, according to this theory, all this is taking place on the surface of the earth, one must consider the destructive effect of ultraviolet radiation on any biologically active structure.
Clearly, the volcanic-rim theory does not advance the cause of chemical evolution, for it represents a dead-end approach to the problem.
Which came first, the chicken or the egg?
All chemical evolutionary scenarios require the pre-biotic production of informational macromolecules. An important question to decide, however, is which type of information biopolymer evolved first, protein or nucleic acid? Proteins are the catalysts of biochemical processes, whereas nucleic acids contain the genetic information for specifying the sequence of amino acids in molecules. In living matter nucleic-acid formation occurs by enzyme catalysis, and protein synthesis is impossible without nucleic acids. Therefore, evolutionists have to solve a puzzle which resembles the question, "Which came first, the chicken or the egg?"
Until recently some theoreticians favored the notion that protein molecules were replicated directly in the absence of nucleic acids, until proteins "invented" nucleic acids. Others felt that nucleic acids were the first biopolymers formed, and they in turn "developed" protein synthesis. A third approach suggested that proteins and nucleic acids co-evolved independent of one another.
These alternatives do not explain satisfactorily the origins of protein and nucleic-acid duplicating systems. For this reason, the discovery that certain types of ribonucleic acids had enzymatic activity was quickly adopted into the chemical evolutionary scenario (13).
In eucaryotic cells, processing of ribonucleic acids often includes the removal of specific intervening nucleotide sequences called "introns" from the RNA molecules. It was found that the intron sequences in the ribosomal RNA of the organism Tetrahyrnena therrnophila spliced themselves without the cooperation of any protein. Moreover, this piece of RNA molecule exhibits true enzymatic activity in that it catalyzed the sequence-specific hydrolysis of certain pieces of other RNA molecules. Introns in fungal mitochondria and in nuclear RNA of higher animals have been also found to self-splice.
Enzymatically active RNAs are called "ribozymes." Their properties combine the most desirable elements of both proteins and nucleic acids. It is not surprising that ribozymes are rapidly taking the center stage among evolutionists as potentially the most likely biomolecules to have been the precursors of Iiving matter, or in other terms, to be both "the chicken and the egg" at the same time.
The difficulties with the ribozyme hypothesis are manifold. Before the existence of RNA in a pre-biotic environment can be postulated, a supply of ribonucleotides- the monomers of RNA -- is needed. Pre-biotic synthesis of ribose can only occur from the polymerization of fairly high concentrations of formaldehyde (0.01M or greater) in alkaline conditions. This reaction yields a mixture of different sugars, ribose being a minor component.
Condensation of ribose with adenine or guanine in the absence of enzymes yields a mixture of unnatural nucleosides (13). Phosphorylation of nucleosides to nucleotides under pre-biotic conditions has not been demonstrated. Condensation of ribonucleotides to oligoribonucleotides in a pre-biotic environment has difficulties similar to those found for the condensation of amino acids to form peptide bonds, with the added problem of having to form 3' to 5' phosphodiester linkages. [There are nine different ways that two ribonucleotides can be linked by a phosphodiester linkage. Only one of these linkages is 3' to 5', although reasons for this linkage being preferred in nature exist (13a).]
Alternative chemical evolutionary scenarios
Some evolutionists have recognized many of the difficulties mentioned above. They observe the high degree of complexity of contemporary organisms and admit the seemingly impossible task of offering a plausible explanation. However, since life is present on Earth, and some sort of mechanistic explanation for its existence is demanded, they continue to search for satisfactory theories.
Dr. Cairns-Smith, a proponent of a new approach to the problem of chemical evolution, points to a seemingly impossible formation in nature, such as an arch of stones (Figure 2). How such an arch could have formed one stone at a time requires a great deal of explaining.
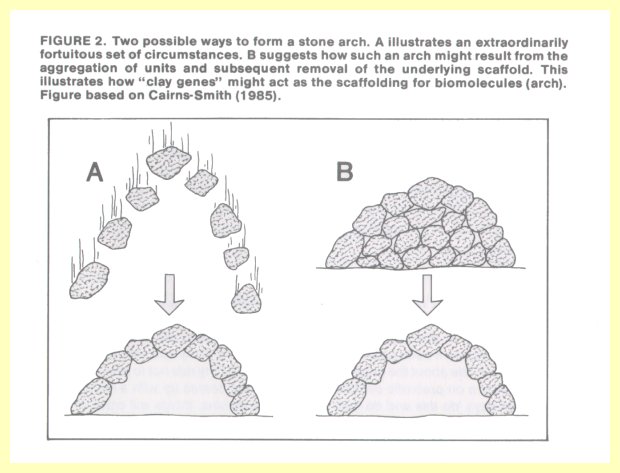
But if we assume that it was the top layer of stones of a round pile, and somehow the "scaffolding" below the top layer was selectively removed, then we have a reasonable explanation.
Attention is called, for example, to crystals of kaolinite (made of layers of aluminum atoms bound in a network of oxygen and silicon atoms). In any given region, the aluminum atoms are positioned in one of three possible arrangements. Such a structure could hold immense amounts of information, which could even be replicated if the relative position of the aluminum atoms is reproduced in each succeeding layer. These structures could behave as "clay genes" which carry genetic information and which, according to Dr. Cairns-Smith, could act as a scaffolding on which present-day biomolecules of RNA and DNA could form (14).
The scaffold theory bypasses the nitty-gritty details of how a living cell can come into existence. It tries to show a way by which information may be transferred in the absence of a biological transfer system. It does not answer where nor how the information originates, neither does it attempt to answer the most obvious question of how the process from inorganic clay to organic polymers occurs. It is essentially an armchair exercise, devoid of experimental support.
A group of evolutionists who cannot envision the evolution of living matter on Earth proposes that life evolved elsewhere in the universe and was imported accidentally or purposefully from outer space. Panspermia was proposed last century as an explanation for life after Pasteur disproved the spontaneous generation of life. It remained quite popular (15), until the organic-soup theory took over in the 1950s. With a fuller appreciation of the difficulties of the organic-soup theory, panspermia is again gaining in popularity.
This theory is essentially an admission of failure to give a convincing naturalistic account for the origin of life on Earth. It pushes the problem out of the realm of experimentation and gives up on suggesting how life could have come about.
Max Delbruck, a confirmed evolutionist, has observed:
"In recent years various theories have outlined the possible connections between molecular selection, natural selection, and irreversible thermodynamics in this prebiotic biochemical trial process. While all these theories seem quite plausible and very intelligent, in my opinion they tell us very little about the origin of life. I have made it my rule not to read this literature on prebiotic evolution until someone comes up with a recipe that says 'do this and do that, and in three months, things will crawl in there.' When someone is able to create life in a shorter time than was originally taken by nature, I will once more start reading that literature" (16).
Why life cannot arise spontaneously
Some general considerations take the topic of the origin of life beyond listing various theories of chemical evolution and a discussion of their inadequacies. First, there is the tacit assumption by evolutionists that matter possesses some sort of internal drive which pushes it to self-organize into living structures. It is as if molecules constituting biopolymers would confer some sort of benefit to their constituent atoms.
There is no evidence that this is the case. Atoms and molecules respond to only one type of drive; that is, to exist in the lowest possible state of energy. Biomolecules are examples of exactly the opposite; they are complexes of atoms in a high-energy state. If atoms had a choice, they would rather get out of being part of the high energy configurations called proteins and nucleic acids.
All mechanistic explanations of origins have two deficiencies. One difficulty is in explaining the source of biological information, which ultimately dictates the structure and function of biopolymers. It is clear that chance cannot provide this information.
A second consideration which renders all mechanistic explanations invalid is that life processes are non-equilibrium events. If by chance all necessary biopolymers and small metabolites could have been produced in the primordial environment, brought together and enclosed in a membrane, a non-living cell would be the result. In the very process of assembly, reactants and their catalysts would be brought together, providing opportunity for individual chemical reactions to reach equilibrium.
There is such a concentration of living organisms on Earth's surface that it is difficult to locate any area that is sterile. Obviously, life had to start somehow. The existence of a supernatural Intelligence who is capable of designing and creating the various living organisms found on Earth is inconceivable to the modern secular mind which is accustomed to explaining all phenomenon by natural processes. But this is precisely the lesson to be learned from our chemical evolutionary efforts. Our inability not only to create living matter but even to suggest how such could come into existence forces us to admit that the existence of life demands the existence of a Creator.
* Published originally as: Javor, G.T.: Origin of life: a look at late 20th Century thinking.
Origins 14:7-20, 1987REFERENCES
(1) Exodus 20:11.
(2)Ingraham, J.L., O. Maaloe and F.C. Neidhardt. 1 983. Growth of the bacterial cell. Sinauer Associates, Inc., Sunderland, Massachusetts, p. 46.
(3)Delbruck, M. 1986. Mind from matter?. Blackwell Scientific Publications, Palo Alto, California, p. 31.(4)Dickerson, R.E. 1978. Chemical evolution and the origin of life. Scientific American 239(3):70-86.
(5)Alberts, B., D. Bray, J. Lewis, M. Raff, K. Roberts, and J. Watson. 1983. The molecular biology of the cell. Garland Publishing Inc., New Yorkand London, p. 11.
(6)(a) Oparin, A.I. 1924. Proiskhozdenie zhizni. Izd. Moskovshii Rabochii, Moscow. English translation by A. Synge printed in J.D. Bernal. 1967. The origin of life. World Publishing Co., Cleveland and New York, pp. 1 99-234. English translation from the 1936 Russian edition by S. Morgulis. 1938. Origin of life. Macmillan Publishers, New York; (b) Haldane, J.B.S. 1929. The origin of life. The Rationalist Annual. Reprinted in J.D. Bernal. 1967. The origin of life. World Publishing Co., Cleveland and New York, pp. 242-249.
(7)Miller, S.L. 1953. A production of amino acids under possible primitive earth conditions. Science 117:528-529.
(8)Thaxton, C.B., W.L. Bradley, and R.L. Olsen. 1984. The mystery of life's origin: reassessing current theories. Philosophical Library, New York. Chapter 3 reviews this topic.
(9)Fox, S.W. and K. Dose. 1977.2nd ed. Molecular evolution and the origins of life. Marcel Dekker Publishing Co., New York.
(10)(a) Pollack, J.B. and D.C. Black. 1979. Implications of the gas compositional measurements of Pioneer Venus for the origin of planetary atmospheres. Science 205:56-59; (b) Pinto, J.P., G.R. Gladstone and Y.L. Yung. 1980. Photochemical production of formaldehyde in Earth's primitive atmosphere. Science 21 0:183-185.
(11) Reference 8, p. 78.
(12)Pocklington, R. 1971. Free amino-acids dissolved in North Atlantic Ocean waters. Nature 230:374-375.
(13)Orgel, L.E. 1986. RNA catalysis and the origins of life. Journal of Theoretical Biology 123:1 27-149. (13a) Usher, D.A. and McHale, A.H. (1976). Hydrolytic stability of helical RNA: a selective advantage for the natural 3',5'-bond. Proc. Nat. Acad. Sci. U.S.A. 73: 2174-2179.
(14)Cairns-Smith, A.G. 1985. The first organisms. Scientific American 252(6):90-100.
(15)Arrhenius, S.A. 1908. Worlds in the making. Harper and Row Publishing Co., New York.
(16) Reference 3, p. 33.